ABOUT
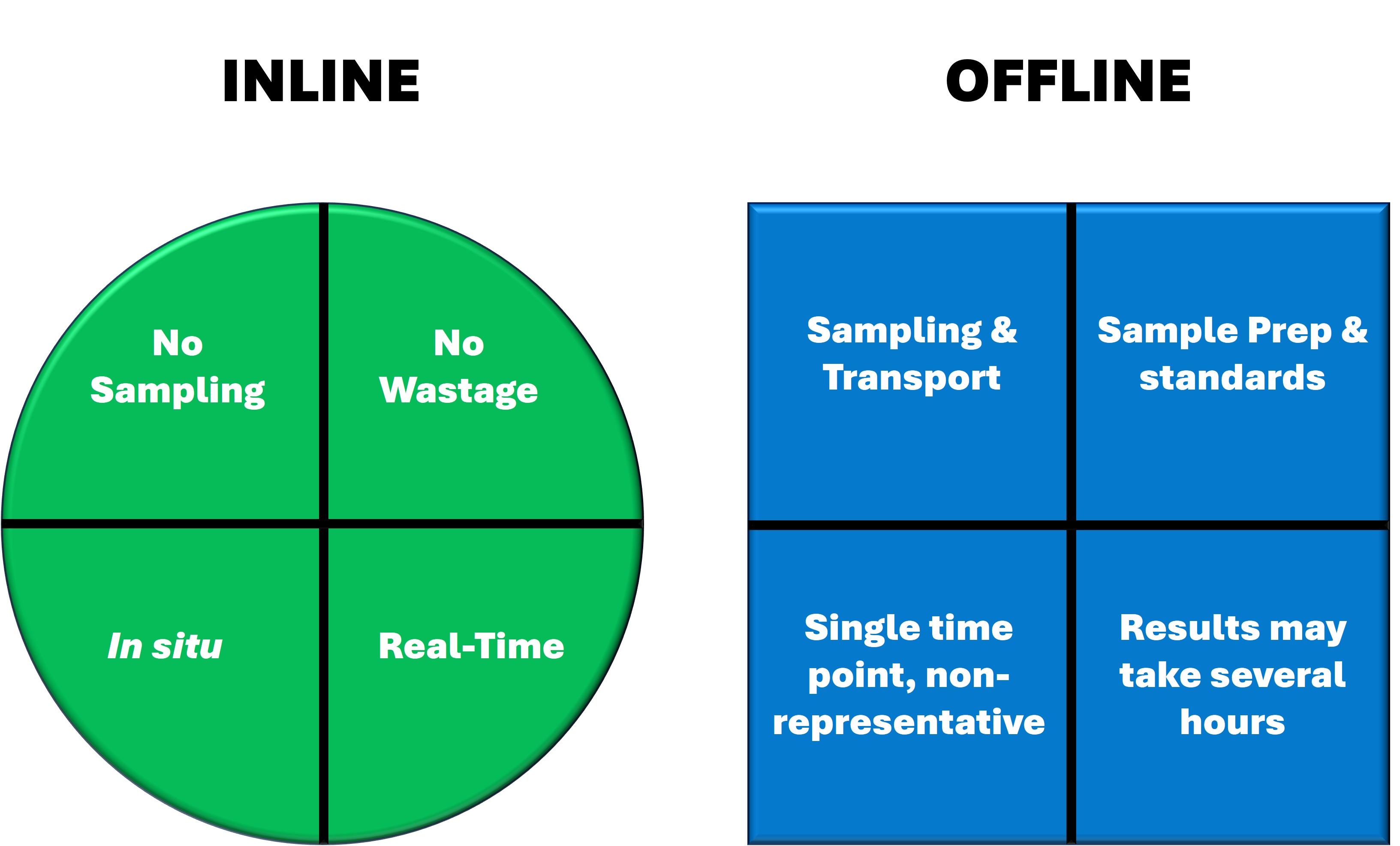
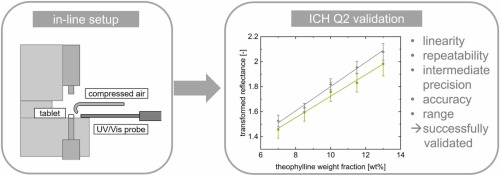
In-line spectroscopy has numerous applications in the manufacture of drug substances and OSD drug products. ColVisTec’s industrial spectrometers are designed for robustness and reliability and can support pharmaceutical manufacturing campaigns by providing 100% product analysis and documentation.
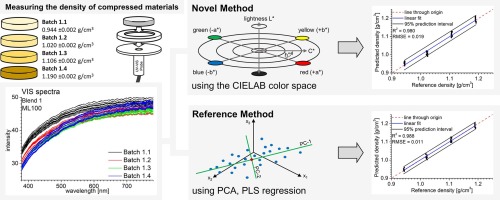
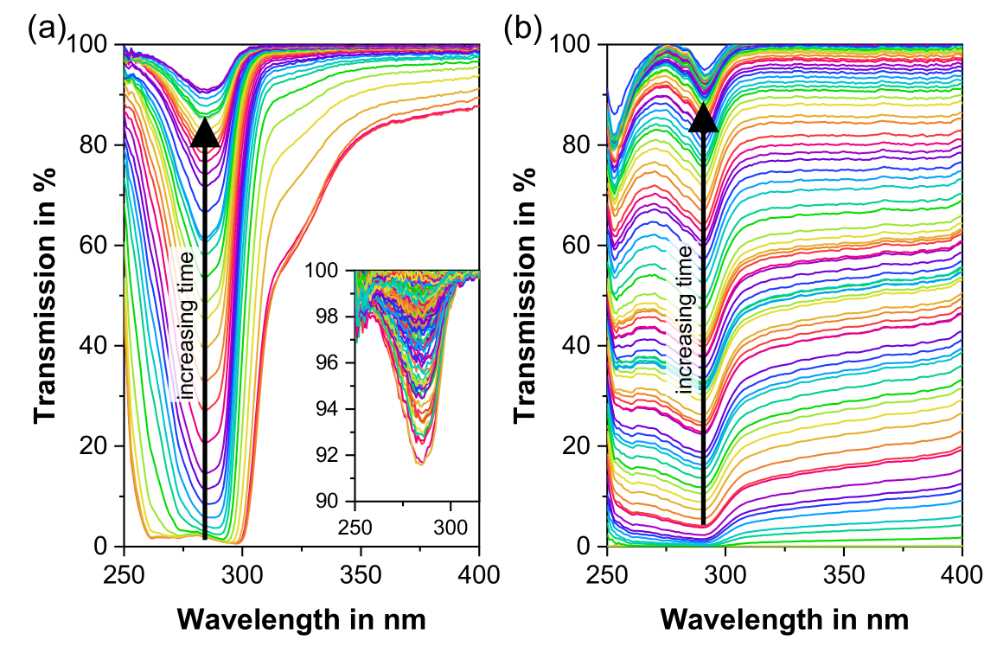
Our spectroscopic techniques can be applied to solids, liquids, solutions, melt phases and amorphous solid dispersions, including intermediate and finished products. Monitor and quantify water-content, API content, crystallinity & dispersion, API degradation, tablet density and hardness - all in real-time.
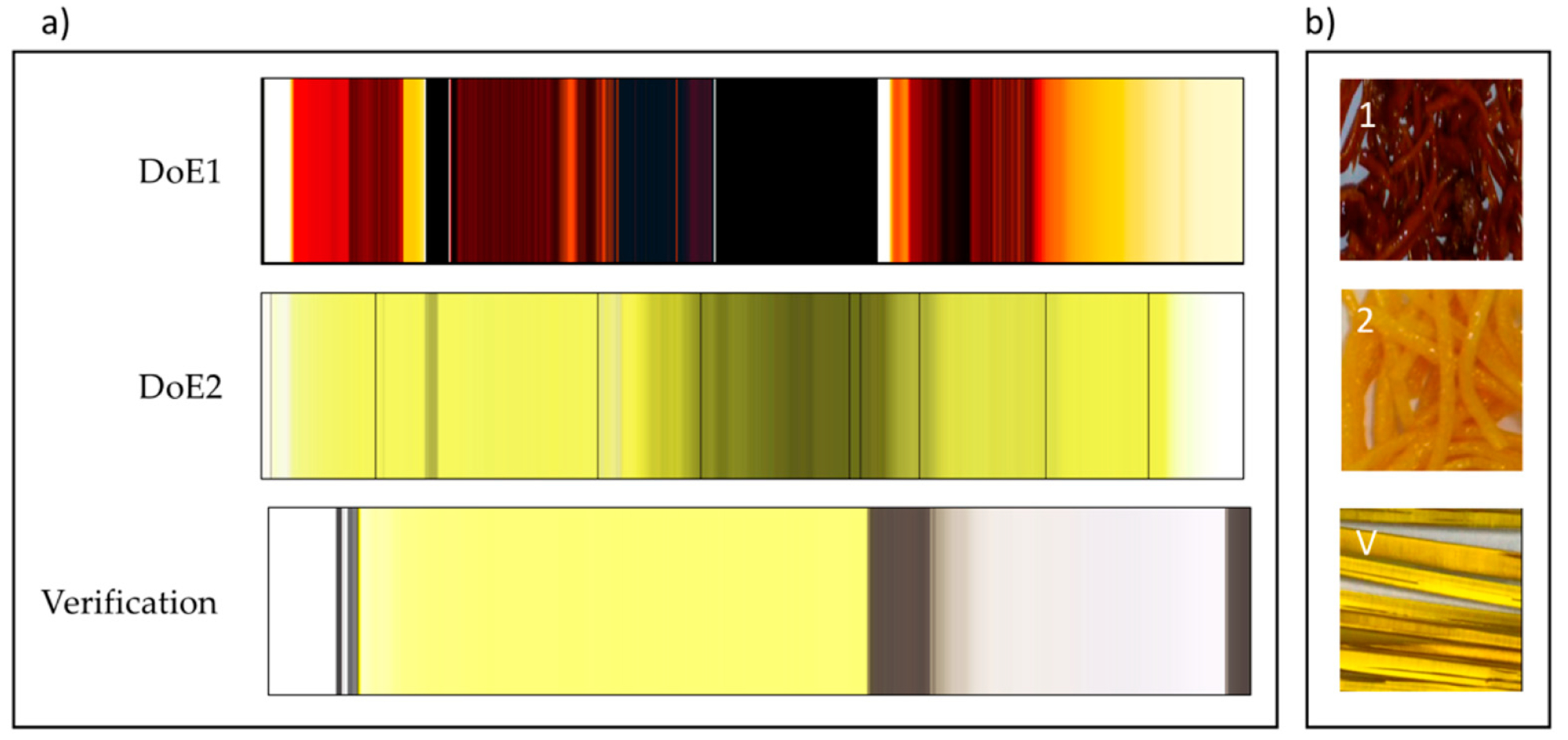
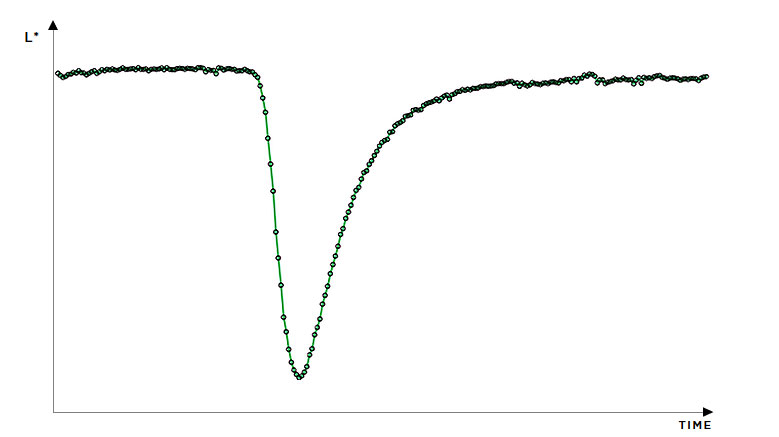
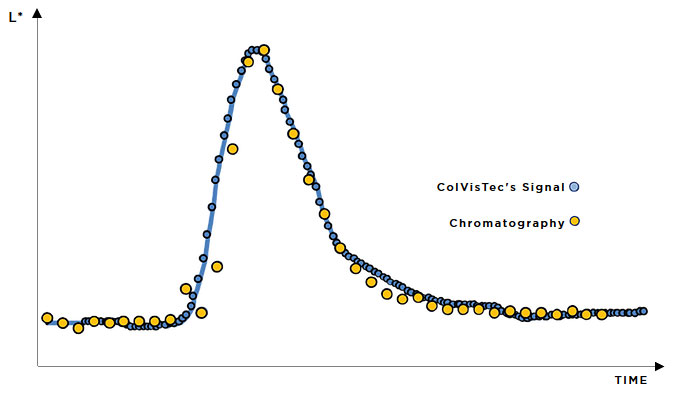
In-line monitoring is an invaluable tool in process development and for rapidly exploring the design space. For ASD hot-melt extrusion processes, UV/Vis can characterise critical process parameters relating to the extruder and peripheral equipment (e.g. residence time, feed rate & consistency, drug dissolution & homogenisation).